Gye Young Park, MD LAB
Macrophage and Dendritic cell research in lung diseases Heading link
Lab News:
Our lab received a new NIH R21 award to study the Heterogeneity and cellular hierarchy of lung cDC2 cells.
Our lab has investigated molecular and cellular mechanisms of immune cells with a more holistic understanding of how these mechanisms work in actual patients. Our studies adopt translational tools based on human samples and apply the data to animal models relevant to human diseases. This approach has resulted in a number of high-quality publications on the phenotypic heterogeneity of immune cells and their transcriptional and posttranslational gene regulation, eventually showing how they modulate pulmonary diseases. More recently, this work has led to an interest in the cellular heterogeneity of macrophages and dendritic cells in asthma. Our lab established the techniques required to analyze the phenotypes and genotypes of innate immune cells, including flow cytometry, sc-RNA-seq, and mass cytometry (CyTOF). To evaluate the clinical relevance of the data from animal models, we run the IRB-approved human translational protocol for bronchoscopic provocation with the sensitized allergen. With the network of collaborators both inside the campus and beyond, our lab will continue to focus on immune cell biology in the lung to understand human lung diseases better.
Selected Key Publications Heading link
- Moon HG, Kim SJ, Kim KH, Kim YM, Rehman J, Lee H, Wu YC, Lee SY, Christman JW, Ackerman SJ, Kim MH, You SY, Park GY. CX3CR1+macrophage facilitates the resolution of allergic lung inflammation via interacting CCL26. Am J Respir. Crit. Care Med. 2023 Jun 1;207(11):1451-1463. PubMed PMID: 36790376; PubMed Central PMCID: PMC10263139.
- Moon HG, Eccles JD, Kim SJ, Kim KH, Kim YM, Rehman J, Lee H, Kanabar P, Christman JW, Ackerman SJ, Ascoli CJ, Kang H, Choi HS, Kim M, You SY, Park GY. Complement C1q is essential aeroallergen sensitization via CSF1R+ conventional dendritic cell 2. Allergy Clin. Immunol. 2023 Nov;152(5):1141-1152.e2. PubMed PMID: 37562753; NIHMSID: NIHMS1924221.
- Wu YC, Moon HG, Bindokas VP, Phillips EH, Park GY, Lee SS. Multi-Resolution 3D Optical Mapping of Immune Cell Infiltrates in Mouse Asthmatic Lung. Am J Respir Cell Mol Biol. 2023 Jul;69(1):13-21. PubMed PMID: 37017484; PubMed Central PMCID: PMC10324044.
- Moon HG, Kim SJ, Jeong JJ, Han SS, Jarjour NN, Lee H, Werner SL, Chung S, Choi HS, Natarajan V, Ackerman SJ, Christman JW, Park GY. Airway Epithelial Cell-Derived Colony Stimulating Factor-1 Promotes Allergen Sensitization. Immunity. 2018, 49(2):275-287. PMID: 30054206; PMCID: PMC6594049. DOI: https://doi.org/10.1016/j.jaci.2018.09.010.
- Moon HG, Kim SJ, Lee MK, Kang H, Choi HS, Harijith A, Ren J, Natarajan V, Ackerman SJ, Christman JW, Park GY. Colony stimulating factor 1 and its receptor are new potential therapeutic targets for allergic asthma. Allergy. 2020 Feb;75(2):357-369. PMID: 31385613; PMCID: PMC7002247.
- Lee YG, Jeong JJ, Nyenhuis S, Berdyshev E, Chung S, Ranjan R, Karpurapu M, Deng J, Qian F, Kelly E, Jarjour N, Ackerman SJ, Natarajan V, Christman, JW, Park GY. Recruited Alveolar Macrophages, in Response to Airway Epithelial-derived MCP-1/CCL2, Regulate Airway Inflammation and Remodeling in Allergic Asthma. Am J Respir Cell Mol Biol. 2015, 52(6):772-84.
- Park GY, Lee YG, Berdyshev E, Nyenhuis S, Du J, Fu P, Gorshkova IA, Li Y, Chung S, Karpurapu M, Deng J, Ranjan R, Xiao L, Jaffe HA, Corbridge SJ, Kelly EA, Jarjour NN, Chun J, Prestwich GD, Kaffe E, Ninou L, Aidinis V, Morris AJ, Smyth SS, Ackerman SJ, Natarajan V, Christman JW. Autotaxin production of lysophosphatidic acid mediates allergic asthmatic inflammation. Am J Respir. Crit. Care Med. 2013, 188(8); 928-940.
Research Heading link
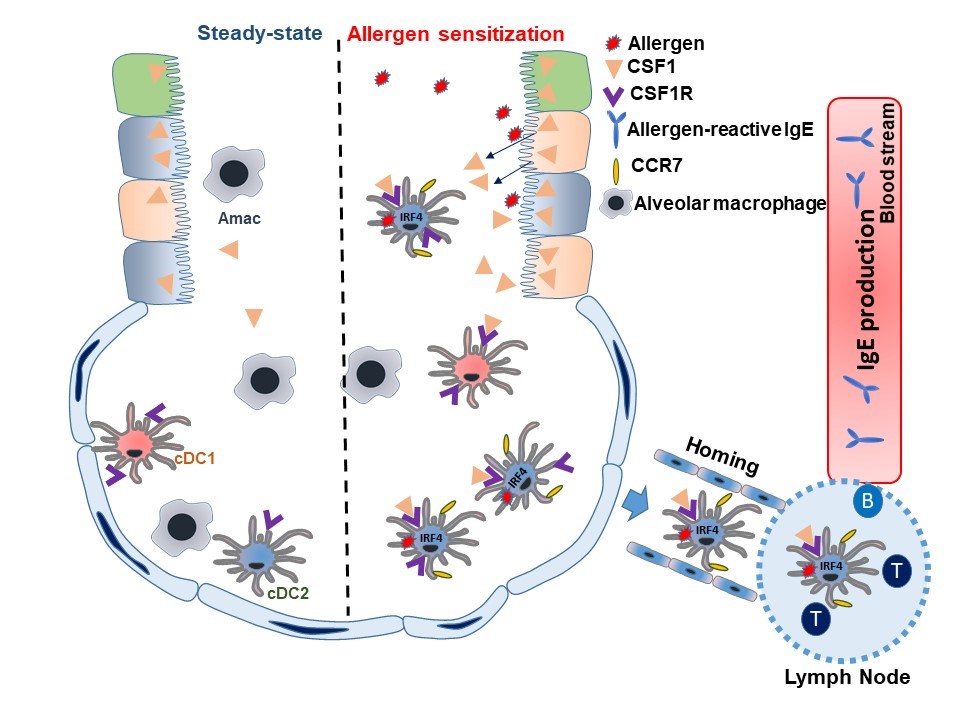
Name of the project: Lung-resident dendritic cell type 2 and allergic asthma.
Brief Description: Sensitization against aero-allergens such as house dust mite and pollen, resulting in elevated specific immunoglobulin E (IgE) levels, represents an early step in the development of allergic asthma. Before our study, there was consensus that airway dendritic cells (DCs) played a key role in taking up and processing inhaled allergen, migrating to the regional lymph node (LN), and presenting antigen to T- and B-cells to establish allergen-specific IgE production, Th2-mediated allergic inflammation, and allergen-specific immunological memory. Previous studies have shown that type-2 conventional dendritic cells (cDC2s), but not cDC1s or monocytic DCs, are essential for sensitization to aero-allergens. However, it remained unknown which cDC2 subsets were involved in this process and how cDC2s were activated to recognize inhaled allergen and promote Th2-mediated immunity.
We previously reported that airway epithelial cells (AECs) are master regulators of the airway micro-environmental milieu through the secretion of various chemokines and cytokines, which recruit inflammatory cells and initiate lung inflammation. This project discovered CSF1as a new member of epithelium-derived alarmins. Both in mice and in humans – the latter under our model of bronchoscopic provocation with sensitized allergen – we found that CSF1 levels were marked increased in bronchoalveolar lavage following allergen challenge. We also discovered the presence of alveolar cDCs, which increased in number following allergen challenge under this model. Our study further revealed that AEC-derived CSF1 binds CSF1R on CSF1R+cDC2s recruited to the airway by allergen exposure. Notably, we found that CSF1 binding with CSF1R enhanced the expression of CCR7 in the CSF1R+cDC2 subpopulation. CCR7 is critical for DC migration to the LN and subsequent antigen presentation. This finding is consistent with other recent reports showing that CSF1 secreted locally by structural cells regulates the functions and survival of myeloid lineage cells.
Research Heading link
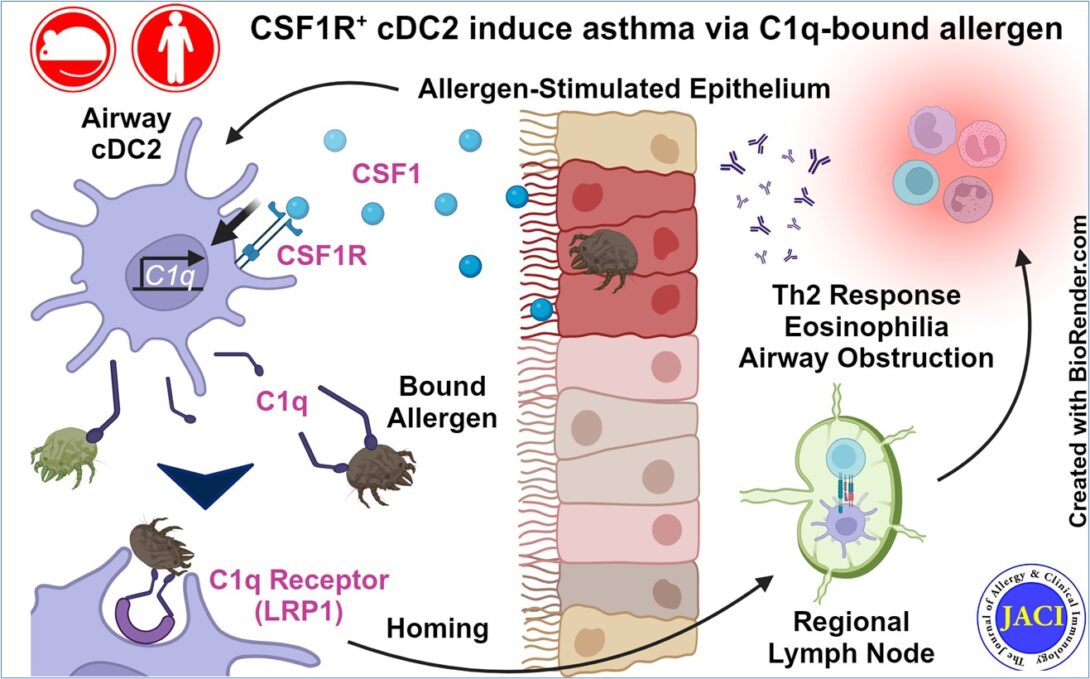
After publication of the results outlined above, we extended this study further to focus on the mechanisms involved in allergen uptake by cDC2s. Initial work was driven by single cell RNA sequencing (scRNAseq) data indicating that CSF1R+cDC2s were enriched for complement component 1q (C1q) expression. We were then able to show that CSF1 stimulation induces C1q transcription and subsequent protein secretion into the alveoli, which was readily measured in BAL from humans and mice following allergen challenge. To our surprise, we next discovered that C1q has direct affinity for dust mite allergen, which we demonstrated by a surface plasmon resonance (SPR) binding assay. Returning to our scRNAseq data for insight, we noted that CSF1R+cDC2s were also enriched for the C1q receptor LRP1 (aka CD91). Follow-up experiments demonstrated that cDC2s capture C1q-bound allergen via LRP1. This interaction could be disrupted by genetic knockout models for either C1q or LRP1, or by antibody blockade of the receptor. Crucially, intervention on this pathway at any step significantly abrogated downstream Th2 cytokine production, eosinophilia, and airway inflammation in our mouse model of asthma. As a final step, we demonstrated strong transcriptional homology between the murine cDC2 subset implicated and a DC subset evident in a publicly available human lung scRNAseq atlas.
In summary, our studies have shown that AEC-secreted CSF1 induces DC-mediated allergen capture via C1q/LRP1 and subsequent trafficking to the draining LN. This cascade leads to the development of adaptive immune response culminating in allergic airway inflammation and asthma. Our work has contributed significantly to the field by discovering that the specific population of CSF1R+cDC2s serves as the key cDC2 subset involved in allergen sensitization, and additionally clarifies its mechanism of allergen uptake. This study highlights how AECs regulate DC function in the airway and illustrates how DCs serve as an essential bridge between innate and adaptive immune responses by mobilizing and presenting allergen to adaptive immune cells.
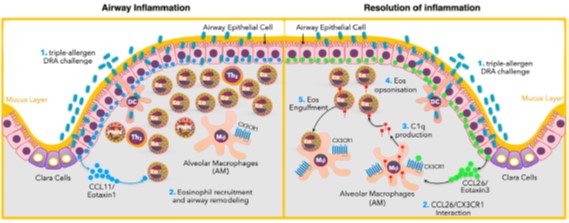
Name of the project: Mechanism of CX3CR1+ macrophage-mediated resolution of eosinophilic allergic lung inflammation Heading link
Name of the project: Mechanism of CX3CR1+ macrophage-mediated resolution of eosinophilic allergic lung inflammation
Brief Description: Asthma is a major healthcare burden to patients, families, and societies. The management of asthma is far less than ideal, in part due to our incomplete understanding of cellular and molecular processes of asthmatic inflammation. This project seeks to determine the novel mechanism of the resolution of allergic inflammation, specifically focusing on a specialized cell type that is responsible for clearing inflammatory cells and their debris to facilitate the resolution of inflammation. Eosinophilic allergic lung inflammation such as asthma is enriched with recruited tissue eosinophils. Most tissue eosinophils undergo apoptotic cell death, which needs to be cleared as the inflammation enters the resolution phase. Tissue macrophages are known to engulf dying cells to restore local hemostasis. However, the mechanisms for how tissue homeostasis is restored after eosinophilic lung inflammation remains largely unknown as to which tissue macrophages are involved in the process. Using human bronchoalveolar lavage samples from patients with asthma and the murine model of allergic lung inflammation, we find that CX3CR1-expressing macrophages engulf eosinophils in allergic asthma. Sc-RNA-seq data show that CX3CR1+ macrophages highly express C1q family genes. Our data show that airway epithelial cell-derived CCL26 avidly binds CX3CR1, and CCL26 binding to CX3CR1 promotes C1q secretion from CX3CR1+ macrophages and enhances their eosinophil clearance. The depletion of CX3CR1+ macrophages leads to the prolonged clearance of tissue eosinophils. These data suggest that CX3CR1+ macrophages play a critical role in clearing tissue eosinophils in asthma.
CSF1 Receptor-Mediated Tumor Microenvironment in Lung Cancer Heading link
Name of the project: CSF1 Receptor-Mediated Tumor Microenvironment in Lung Cancer
Brief Description: Thanks to the collaborative efforts of immunologists and oncologists, cancer immunotherapy and its immunologic research made clinical and scientific breakthroughs in the treatment of lung cancer. Our lab investigates the role of tumor-infiltrating myeloid cells such as macrophages and dendritic cells (DCs) and seeks a new approach for myeloid cell-mediated cancer immunotherapy. The microenvironmental milieu determines the phenotype of myeloid cells. Our lab reported that Colony Stimulating Factor 1 (CSF1), one of key mediators of the microenvironment, is critical for determining the immunologic function of a DC subset. It has been well known that tumor cells secrete CSF1 to alter their microenvironment, yet the mechanism of action is not fully elucidated. In ongoing clinical trials, several agents targeting CSF1 receptor (CSF1R) are being tested for cancer treatment. In this project, we study a new role of tumor-produced CSF1 in altering tumor immune microenvironment toward tumor progression by producing autotaxin (ATX) which increases the number of protumoral myeloid cells such as macrophages and DCs. ATX, also known as lysophospholipase D, is secreted extracellularly and enzymatically generates lysophosphatidic acid (LPA), the most abundant phospholipid in body fluid. LPA is well known to stimulate cellular proliferation, migration, and survival for myeloid lineage cells. The project is based on our two new critical discoveries on myeloid cell biology; (1) ATX is highly expressed in myeloid cells under the control of CSF1 and its receptor (CSF1R) activation, (2) ATX regulates the size of the residential population of lung myeloid cells in the CSF1R-dependent manner (CSF1R-ATX pathway). Together these findings have led to the hypothesis that tumor-produced CSF1 stimulates protumoral myeloid cells to secrete ATX to increase the number of protumoral macrophages and DCs. To validate the hypothesis, we employ the approaches for proof-of- concept by utilizing the novel transgenic mice that have the loss or excess of the target genes. The clinical arm exploits clinical samples from patients with lung cancer to verify the proof-of-concept. Lastly, in the translational arm, we examine the therapeutic potentials of interfering CSF1R-ATX pathway in the mouse lung cancer model by adopting a state-of-art nano-delivery system.
