Levitan Irena, PhD LAB
Membrane Biophysics and Cytomechanics in Vascular Biology
Lab News:
Sang Joon Ahn et al, Hypertension 2022
Cholesterol-Induced Suppression of Endothelial Kir Channels Is a Driver of Impairment of Arteriolar Flow-Induced Vasodilation in Humans
https://pubmed.ncbi.nlm.nih.gov/34784737/
New Methods and Sensors for Membrane and Cell Volume Research
https://www.sciencedirect.com/bookseries/current-topics-in-membranes/vol/88/suppl/C
On, in, and under membrane. Curr Top Membr. 2021
Our goal is to elucidate biophysical molecular mechanisms of vascular dysfunction in hypercholesterolemia and dyslipidemia, focusing on ion channels, cytomechanics and mechanotransduction. Our work provided fundamental new understanding of cholesterol regulation of ion channels, paradoxical relationship between membrane and cellular biomechanics and provided significant novel insights into endothelial response to flow.
On a mechanistic level, we focus on specific lipid-protein interactions addressing the following questions:
(1) How cholesterol interacts with the channel proteins and how these interactions govern channel function;
(2) How cholesterol-induced suppression of ion channels alters endothelial and vascular function;
(3) How incorporation of oxidized lipids into the membrane initiates endothelial stiffening;
(4) How endothelial stiffening contributes to vascular dysfunction.
These questions are addressed by a combination of molecular modeling, molecular and cellular biology, electrophysiology, imaging and atomic force microscopy. In terms of the disease models, we study atherosclerosis, hypertension, and abnormal angiogenesis.
Selected Key Publications Heading link
- Epshtein, Y., Chopra, A., Rosenhouse-Dantsker, A. Kowalsky, G., Logothetis D.E. and I. Levitan. Identification of a C-terminus domain critical for the sensitivity of Kir2.1 channels to cholesterol. PNAS, 106(19):8055-60, 2009
- Shentu TP, Singh DK, Oh MJ., Sun S, Sadaat L, Makino A, Mazzone T, Subbaiah PV, Cho M and I Levitan. The role of oxysterols in control of endothelial stiffness. Journal of Lipid Research, 53:1348-58, 2012. Featured in International Innovation Report.
- Rosenhouse-Dantsker A, Noskov S, Durdagi S, Logothetis DE, Levitan I. Identification of novel cholesterol-binding regions in Kir2 channels. Journal of Biological Chemistry, 288(43):31154-64, 2013
- Oh MJ, Zhang C, LeMaster E, Adamos C, Berdyshev E, Bogachkov Y, Kohler EE, Baruah J, Fang Y, Schraufnagel DE, Wary KK, Levitan I. Oxidized LDL signals through Rho-GTPase to induce endothelial cell stiffening and promote capillary formation. J Lipid Res. 57(5):791-808, 2016. Featured in Atlas of Science.
- LeMaster E., Huang RT, Zhang C, Bogachkov Y, Coles C, Shentu TP, Sheng Y, Fancher IS, Ng C, Christoforidis T, Subbaiah PV, Berdyshev E., Qain Z., Eddington DT, Lee J, Cho M, Fang Y, Minshall RD and I Levitan. Pro-atherogenic flow increases endothelial stiffness via enhanced CD36-mediated oxLDL uptake. ATVB.38:64-75, 2018. ATVB Featured Article, Editorial Comment.
- Fancher IS, Ahn SJ, Adamos C, Osborn C, Reardon CA, Getz GS, Phillips SA, and I Levitan. Hypercholesterolemia-induced endothelial dysfunction is rescued by overexpression of endothelial Kir2.1 in resistance arteries. J Am Heart Assoc. 7(5). pii: e007430. 2018. JAHA editorial Comment.
- Fancher IS, Le Master E, Ahn SJ, Adamos C, Lee JC, Berdyshev E, Dull RO, Phillips SA, Levitan I. Impairment of Flow-Sensitive Inwardly Rectifying K+ Channels via Disruption of Glycocalyx Mediates Obesity-Induced Endothelial Dysfunction. Arterioscler Thromb Vasc Biol. 40(9):e240-e255, 2020
Books Heading link
Books
ㅤ
Books

ㅤ
Books

ㅤ
Books

ㅤ
Books

ㅤ
Books

ㅤ
Current Lab Members Heading link
Sang Joon Ahn, PhD

Sang Joon Ahn, PhD
Research Assistant Professor
Natalia Do Couto, PhD
Dana Lazarko
Sara Granados, PhD

Sara Granados, PhD
Postdoctoral Associate
Victor Aguilar, PhD
Chris Wu
Elizabeth Le Maser, PhD

Elizabeth Le Maser, PhD
Postdoctoral Associate
Amit Paul
Research Heading link
Cholesterol Regulation of K+ Channels
Cholesterol-Kir Interactions
Cholesterol is known to regulate multiple types of ion channels but molecular mechanisms and the structural determinants of these effects until recently have been almost completely unknown. Traditionally, it was believed that cholesterol regulates ion channels by altering the physical properties of the membrane lipid bilayer. Our studies pioneered a change in this paradigm by demonstrating that cholesterol-induced suppression of inwardly-rectifying K+ channels (Kir) channels is mediated by direct specific interaction between cholesterol and the channel protein and identified cholesterol binding sites as non-annular hydrophobic pockets in the channel protein (Romanenko et al, 2002, 2004; Fang et al 2006, Epshtein et al. 2009, Rosenhouse-Dantsker 2011, 2012, 2013, 2014, Han et al 2014).
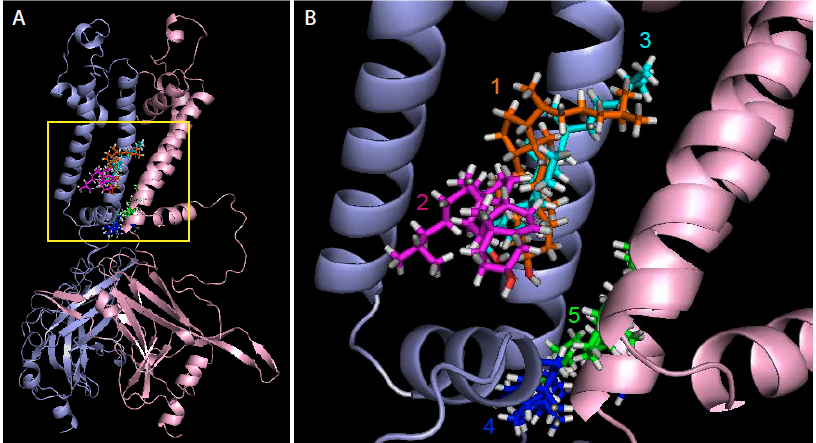
Nik Barbera Heading link
Using MARTINI coarse-grained simulations to explore the dynamic, unbiased migration of cholesterol molecules from the lipid membrane to the Kir2.2 protein, we found that the cholesterol environment surrounding Kir channels forms a complex milieu of different short- and long-term interactions, with multiple cholesterol molecules concurrently interacting with the channel. Furthermore, utilizing principles from network theory, we identified four discrete cholesterol binding sites within the previously identified cholesterol sensitive region, which exist depending on the conformational state of the channel – open or closed (Barbera et al 2018, Biophysical Journal).
Cholesterol-induced Suppression of Endothelial Kir channels is a Major Contributor to Endothelial Dysfunction in Hypercholesterolemia Heading link
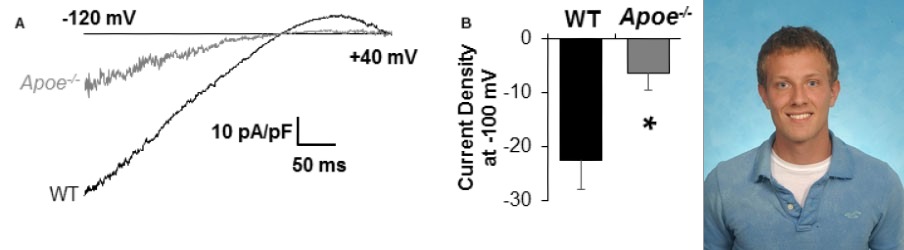
Cholesterol-induced Suppression of Endothelial Kir channels is a Major Contributor to Endothelial Dysfunction in Hypercholesterolemia
We also discovered that Kir channels play a major role in hypercholesterolemia-induced endothelial dysfunction. First, we established that endothelial Kir channels are a major contributor to NO-dependent flow-induced vasodilation. Then, we discovered that the loss of Kir function under hypercholesterolemia results in a significant reduction of flow-induced NO production and flow-induced vasorelazation. Remarkably, endothelial specific overexpression of Kir2.1 recovers shear-stress sensitivity of inwardly-rectifying Kir currents in cells loaded with cholesterol. These findings identify cholesterol-induced suppression of Kir channels as one of the major contributors to endothelial dysfunction in hypercholesterolemia (Fancher et al, 2018, JAHA).
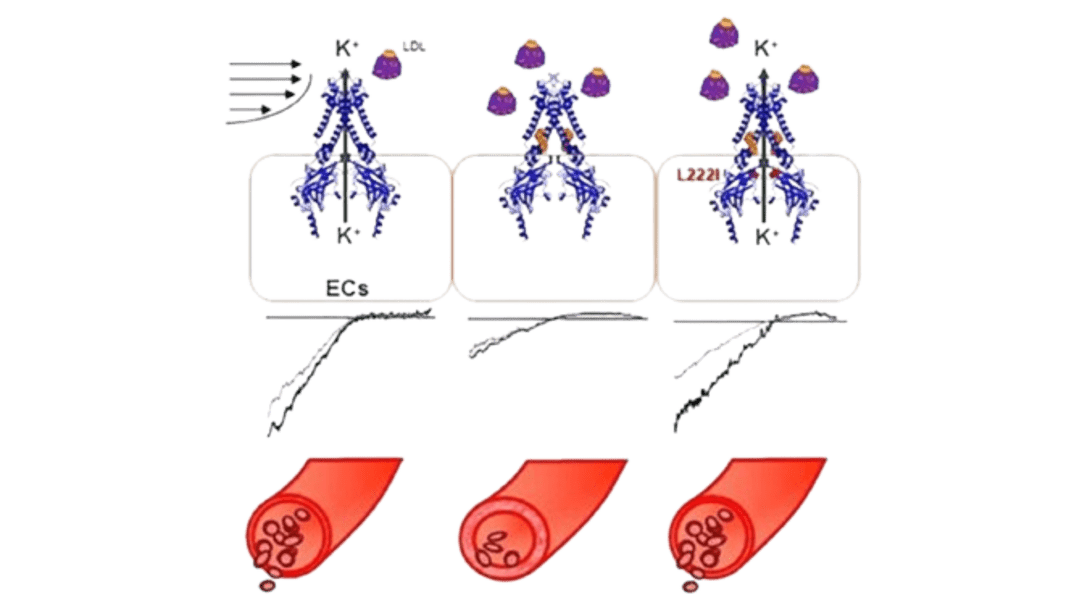
Cholesterol-Induced Suppression of Endothelial Kir Channels Is a Driver of Impairment of Arteriolar Flow-Induced Vasodilation in Humans Heading link

Cholesterol-Induced Suppression of Endothelial Kir Channels Is a Driver of Impairment of Arteriolar Flow-Induced Vasodilation in Humans
-
Cholesterol-induced suppression of endothelial Kir channels is identified as a critical link between hypercholesterolemia and increase in peripheral resistance leading to hypertension.
- Hypercholesterolemia is a major risk factor for vascular dysfunction and hypertension. This study shows that cholesterol-induced reduction of flow-induced vasodilation is due to suppression of Kir2.1, resulting in increasing peripheral resistance. The critical level of LDL that causes Kir2.1 dysfunction is 100 mg/dL low-density lipoprotein (LDL) cholesterol, a recommended target by American Heart Association.
The study presents the first direct evidence that elevated plasma LDL in humans lead to severe suppression of flow-induced vasodilation via the loss of endothelial Kir function, whereas increase in Kir2.1 expression or rendering Kir2.1 to be cholesterol insensitive have a full rescue effect. (Ahn et al, 2022, Hypertension).
Impact of Dyslipidemia on Endothelial Biomechanics Tzu-Pin Shentu Paradoxical relationship between lipid order and cell stiffness Heading link
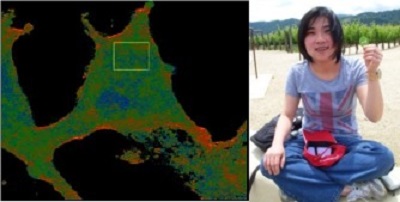
Impact of Dyslipidemia on Endothelial Biomechanics
Paradoxical relationship between lipid order and cell stiffness
Cell stiffness or deformability is a fundamental property that is expected to play a major role in multiple cellular functions. It is well known that cell stiffness is dominated by the intracellular cytoskeleton that together with the plasma membrane forms a membrane-cytoskeleton envelope. However, our understanding of how lipid composition of plasma membrane regulates physical properties of the underlying cytoskeleton is only starting to emerge. Initially, our hypothesis was that cholesterol loading of endothelial cells should increase endothelial stiffness and impair the ability of endothelial cells to respond to shear stress. Our first surprise, however, was to discover that cholesterol loading has no effect on EC stiffness whereas it is cholesterol depletion that induces EC stiffening. Furthermore, we discovered that plasma dyslipidemia results in an increase in stiffness of aortic endothelial cells and we propose that endothelial stiffening is the key early step in endothelial dysfunction that leads to the disruption of the endothelial barrier and alters the sensitivity of endothelial cells to shear stress forces. These findings uncover a fundamentally novel relationship between the physical properties of the membrane lipid bilayer and cell stiffness (Byfield et al 2004, 2016, Shentu et al 2010, 2012).
Manuela Ayee Heading link
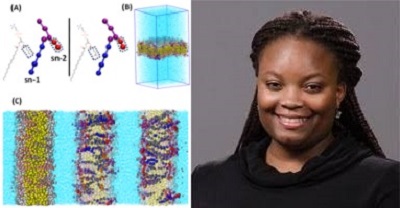
We also established an inverse relationship between the impact of specific oxidized phospholipid derivatives of arachidonic acid (PGPC and POVPC) on the structure of membrane lipid bilayers and on the endothelial elastic modulus. We have shown using molecular dynamics simulations that, similarly to oxysterols, oxidized phospholipids incorporated in a multicomponent phosphatidylcholine bilayer result in disruption of lipid packing of the membrane and increase in cellular stiffness (Ayee et al 2017, Biophysical Journal).
Pro-atherogenic flow increases endothelial stiffness of aortas via enhanced CD36-mediated oxLDL uptake Heading link

Pro-atherogenic flow increases endothelial stiffness of aortas via enhanced CD36-mediated oxLDL uptake
Disturbed flow (DF) is well-known to induce endothelial dysfunction and synergistically with plasma dyslipidemia facilitate plaque formation. Little is known, however, about the synergistic impact of DF and dyslipidemia on endothelial biomechanics. Our new study shows that disturbed flow facilitates endothelial CD36-dependent uptake of oxidized lipids resulting in local increase of endothelial stiffness in pro-atherogenic areas of the aorta. We propose that endothelial stiffening plays a major role in predisposing these regions to the development of atherosclerosis (LeMaster et al, 2018, ATVB).
Trainees Heading link
Former Trainees
Former PhD students:
Yun Fang, PhD
Tzu-Pin Shentu, PhD
Myung Oh, PhD
Sang Joon Ahn, PhD
Elizabeth LeMaster, PhD
Nicolas Barbera, PhD
Yedida Bogachkov, PhD
Former Postdoctoral Fellows and Associates:
Dev Singh, PhD
Yulia Epstein, PhD
Johnson Thomas, PhD
Avia Rosenhouse-Dantsker, PhD
Frank Kuhr, PhD
Eric Han, PhD
Manuela Ayee, PhD
Wenjing Feng, MD
Ibra “Drew” Fancher, PhD
