Research Projects
Current Research Heading link
-
Accelerated Chest Pain Workup Equity Task Force
Chest pain evaluations are one of the most frequent reasons adults present to the ED. An ED cardiac work up includes serial lab testing and often leads to post-ED follow-up recommendations, which burden health system capacity. This project involves a retrospective chart review of ongoing clinical care to identify the effects of the implementation of the pathway and troponin assay changes on the evaluation of chest pain at UI Health.
Who is involved:
- Dr. Samuel Wainwright
- Dr. Lauren Smith
- Dr. Ming Jin
- Dr. Shaveta Khosla
Contact
- Shaveta Khosla
skhosl2@uic.edu - Ruth Pobee
rpobee@uic.edu
-
Better Health Through Housing
The Better Health Through Housing (BHH) program is a partnership between the University of Illinois Hospital & Health Sciences System (UI Health) and the Center for Housing and Health (CHH), a subsidiary of the Aids Foundation of Chicago. It is a demonstration pilot that is drawing attention to the nationally-validated Housing First model here in Chicago, with a goal of scaling and sustaining a permanent collective solution among healthcare, government, and housing agencies that will result in a dramatic reduction in the number of chronically homeless. Although the chronically homeless constitute 10-15% of the total homeless population, they account for 80-90% of public costs and utilization. Housing First programs throughout the country have demonstrated it costs society one-third to a half as much to provide supportive housing rather than allowing citizens to remain homeless.
UI Health pays CHH $1,000 per patient per month once a patient has transitioned into permanent support. Housing stock comes from a cooperative of 28 supportive housing agencies representing 150 one-bedroom apartments scattered throughout the city and single-room occupancy (SRO) studio units. This is a collaborative interdisciplinary model that includes hospital social workers, supportive housing case managers, and street outreach workers, with CHH playing a project management and coordination role.
BHH has created a healthcare-to-housing model demonstrating a significant drop in healthcare costs (-21%).
A few of the many lessons learned through the pilot:
- Homelessness is a dangerous health condition.
- The pilot has shown high mortality rates. Four of the 27 expired during the program’s first year and another is in hospice. The homeless in America have life expectancies of 25 years less than the average American.
- There are high rates of head and neck cancers, and traumatic brain injuries.
- The homeless are invisible in the healthcare system. When the program started, we had identified 48 individuals and their healthcare costs were 4.8 times higher than the average UI Health patient. We have now found over 1,325 patients (2008-2017) with 574 current patients who are experiencing homelessness or unstable housing.
- The process for identification, selection, intake, outreach and transition to bridge unit and permanent supportive housing requires intensive care coordination among an interdisciplinary cross-agency care team. Team members include hospital social workers, psychiatrists, ED physicians, outreach workers, supportive case managers, and project managers.
Contact
Steve Brown, MSW, LCSW
sbbrown9@uic.edu - Homelessness is a dangerous health condition.
-
Designing a Hypertension Action Plan for Socially Vulnerable Patients
Purpose:
The purpose of this study was to design, develop and pilot test a tailored human centered hypertension action plan in UI Health patient population.Study Design:
The study consists of two phases: the development phase and the pilot phase. The development phase was used to develop and effectively tailor an action plan for hypertensive patients. The pilot phase involves recruitment of patients to implement the action plan.Who is involved:
- Dr. Heather Prendergast
- Hugh Musick
- Dr. Bhrandon Harris
- Dr. Charles McPherson
- Dr. Shaveta Khosla
- Other valuable partners and collaborators from UI Health
-
Illinois Heart Rescue
Illinois Heart Rescue (ILHR) seeks to increase out-of-hospital cardiac arrest (OHCA) survival rates in the State of Illinois by at least 100%. To accomplish this goal, we have partnered with the Illinois Department of Public Health and existing state-wide quality improvement systems (Get with the Guidelines Stroke, Mission Lifeline) to recruit and train Emergency Medical Services (EMS) systems and hospitals in Illinois to collect quality cardiac arrest data into the CARES database that encourages improvement in local systems of care for sudden cardiac arrest. Our focus is to reduce health disparities in cardiac arrest outcomes using hot spotting to identify and intervene in communities with the highest incidence of cardiac arrest and poorest outcomes.
Since 2013, ILHR has trained over 24,300 community members on Hands Only CPR and AED use through establishing strong partnerships via diverse trainings at schools, hospitals, health associations, cultural centers, and major league sporting events, primarily in identified “hotspot”neighborhoods that have been documented to have the highest incidence of OHCA and the lowest incidence of bystander CPR. ILHR training efforts have resulted in bystander CPR rates in Chicago increasing from 13% in 2013 to 24% in 2015. In this time, Illinois has doubled its survival rate from 4% to 8%.
Contacts
Courtney Schwerin, Executive Director
courtney.ILHR@gmail.com -
Health Screening, Education, Awareness, Linkage to Care
What We Do:
Project HEAL is a screening, education, and linkage to care initiative at UI Hospital and Health Sciences System (UI Health). The high volume of patients seen at UI Health – a large, urban medical center and safety-net provider in Chicago – is an ideal point of contact for patients to be widely screened for treatable diseases, such as HIV, Hepatitis C, Syphilis, and diabetes. Project HEAL is housed in the Department of Emergency Medicine and is a collaborative effort between Emergency Medicine, Infectious Disease, Pathology, Public Health, Hepatology, and Internal Medicine.
Our Vision & Mission – Health, Education, Awareness, & Linkage to Care.
Our vision is proactive and preventive Emergency Medicine that focuses on underserved populations to address health inequities.
Our mission is to develop electronic medical record driven innovations to diagnose and link vulnerable patients seen in the Emergency Department to long-term care and treatment to improve patient outcomes. And further, to engage in public health research, build upon current programs, and establish best practices for social emergency medicine.
Project HEAL Team
- Janet Lin, MD, MPH, MBA
Principal Investigator
Associate Professor, Department of Emergency Medicine, UI Health
Director, International Emergency Medicine and Health Fellowship Program, UI Health - Cammeo Mauntel-Medici, MPH
Associate Director of Project HEAL
Visiting Research Specialist, Department of Emergency Medicine, UI Health
Email: cmedic2@uic.edu - Anjana Maheswaran, MPH
Senior Research Data Analyst
Senior Visiting Research Specialist Project Heal, Department of Emergency Medicine, UI Health - Jasmine Green, MPH
Education and Care Coordination Specialist
Visiting Research Associate II Project Heal, Department of Emergency Medicine, UI Health
Email: jgreen00@uic.edu - Renee Petzel Gimbar, Pharm.D.
Emergency Services Project Leader
Clinical Pharmacist, Dept of Emergency Services & Dept of Pharmacy Practice, UI Health - Andrew Trotter, MD, MPH
Infectious Disease Liaison
Assistant Professor of Clinical Medicine, Infectious Disease Clinician, Division of Infectious Diseases, UI Health - Michelle Martin,Pharm.D., BCPS, BCACP
HCV Treatment Liaison
Clinical Pharmacist, Bobbie and Marvin Fink Family Liver Clinic, UI Health
- Janet Lin, MD, MPH, MBA
-
Strategies to Innovate EmeRgENcy Care Clinical Trials (SIREN) Network
The Strategies to Innovate EmeRgENcy Care Clinical Trials (SIREN) Network is jointly funded by NHLBI, NINDS, DOD, and NCATS. More emergency care research is needed to improve outcomes in leading causes of death and disability, including heart attack, stroke, cardiac arrest and trauma. While we know that early treatment in many of these conditions can significantly improve outcomes, research studies of possibly better or new treatments can be difficult to implement in the emergency department setting. The SIREN program creates a single network in the United States for high quality emergency care research capable of answering such important questions. Participating hospitals and emergency medical services within this network will study patients with neurologic (e.g. stroke, seizure), cardiac (heart attack, congestive heart failure, cardiac arrest), traumatic (car accident, gunshot wounds), pulmonary (asthma, emphysema) and hematologic (sickle cell crisis, acute anemia) emergencies. The SIREN program builds upon both the Resuscitation Outcomes Consortium (ROC) and the Neurologic Emergencies Treatment Trials (NETT) as remarkably successful multiple large-scale clinical trial networks that have helped improve both emergency research processes and care. Trials conducted through these research networks have greater efficiency than similar trials outside these networks. Given this success, the SIREN program seeks to continue support of a network infrastructure that will efficiently conduct at least four randomized, clinical endpoint trials related to emergency medicine over a five-year period.
The purpose of the SIREN Network is to:
- Provide a robust and readily accessible infrastructure for rapid implementation and high quality performance of clinical trials in patients with neurologic, cardiac, respiratory, and hematologic, and trauma emergency events
- Advance emergency medicine by efficiently enabling performance of rigorous comparative effectiveness studies and assessments of novel therapeutic interventions
The University of Illinois at Chicago is part of the Medical College of Wisconsin Hub.
UIC Adult Expansion Sites Include:
- Rush University Medical Center
- John H. Stroger Cook County Hospital
- Sinai Health Hospital
- Advocate Lutheran General Hospital
- Advocate Christ Hospital
- Advocate Illinois Masonic Hospital
- OSF Saint Francis Hospital
Contacts
Marina Del Rios, MD
mdelrios@uic.eduSandra Escobar-Schulz
escobars@uic.edu -
Targeting of Uncontrolled Hypertension in the Emergency Department (TOUCHED)
Project Description
Targeting of Uncontrolled Hypertension in the Emergency Department (TOUCHED) is a two-arm randomized, controlled clinical trial targeting a high-risk emergency department population with persisting evidence of moderately elevated blood pressure (≥140/90 mmHg) at the time of discharge from the emergency department. It has been funded by the National Institutes of Health and will run for 5 years with an enrollment goal of 770 participants.
Rates of uncontrolled hypertension (HTN) in the Chicago area are significantly higher than the US national averages and reflect a significant health disparity especially among minority populations. The prevalence of uncontrolled hypertension (≥140 mmHg systolic or 90 mmHg diastolic) within the University of Illinois Hospital and Health System (UI Health) primary service area is about two-fold that of the USA national rates (69% among Non-Hispanic Blacks and 68.9% among Hispanics). Uncontrolled HTN is a significant contributor to cardiovascular morbidity and mortality and is frequently encountered among patients presenting to the emergency department (ED). EDs serve as the point of entry into the health care system for many high-risk patient populations, particularly minority and low-income individuals. EDs are well situated at the interface between inpatient and outpatient care and can serve as a portal for identifying high-risk individuals and initiating validated screening methods. The TOUCHED study is highly responsive to the call for more research in minority populations particularly African Americans and Hispanics to improve HTN treatment and control.
Who is involved?
- Principal Investigator: Heather Prendergast, MD, MPH, MS
- Co-Investigator: Martha Daviglus, MD, PhD (Participant retention)
- Co-Investigator: Marina Del Rios, MD, MS (Echocardiogram)
- Co-Investigator: Renee Petzel Gimbar, PharmD (Pharmacy consultation)
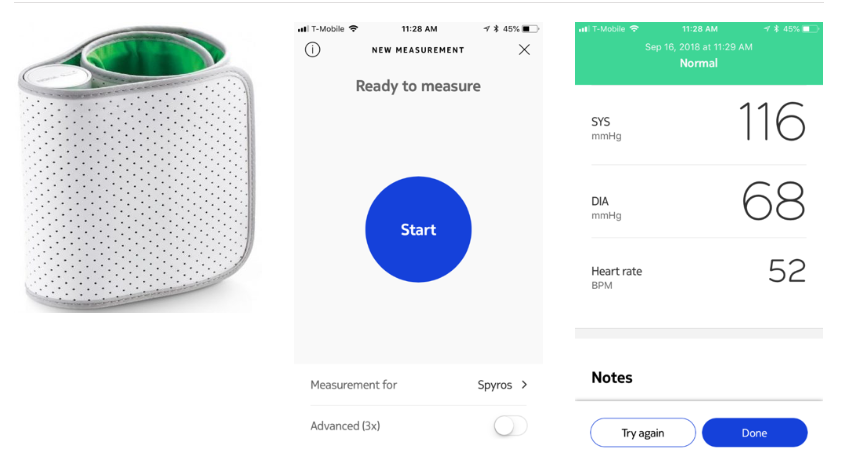
- Co-Investigator: Spyros Kitsiou, PhD (Remote Blood Pressure Monitoring)
- Consultant: Ramon Durazo-Arvizu, PhD (Statistical Analysis)
- Consultant: Barry Carter, PharmD (Pharmacy Consultation)
- Director of Research: Sara Heinert, PhD (Research Assistant Recruitment)
- Project Manager: Brenda Lara, MA
- Project Coordinator: Maya Jackson, MA
Main Objectives:
The primary objective is to determine the effectiveness of an emergency department-initiated Educational and Empowerment (E2) intervention targeting moderately elevated BPs (≥140/90 mmHg) in a predominately minority population with high rates of uncontrolled hypertension by examining mean BP differences between the two trial arms. Secondary objectives include testing whether this ED education and empowerment intervention improves overall rates of BP control (defined as BP < 140/90 mmHg), improvement in cardiovascular risk score, medication adherence (as measured by the Modified Morisky Scale), primary care engagement (measured by compliance with outpatient follow-up appointments), and HTN knowledge improvement (as measured by a validated HTN knowledge survey).
Study Design
Emergency Department patients who have completed their ED evaluation and have been identified for discharge yet still have elevated BPs, will be recruited and randomized into one of two arms: 1) usual care (preprinted discharge instructions and 48-72 hour outpatient referral for primary care follow-up); 2) ED-based E2 intervention with a Post-Acute Care Hypertension Transition–consultation (PACHT-c) by ED clinical pharmacist or APN followed by 48-72 hour referral for primary care follow-up. The Educational and Empowerment (E2) intervention consists of an educational video on HTN, visual clips of age/gender-matched echocardiograms, and blood pressure monitoring kit.
This is novel because it is initiated in the emergency department, is an innovative change to the current care delivery model, utilizes mobile health home BP monitoring, and can decrease the health disparity gaps associated with uncontrolled HTN. Our E2 intervention will educate patients about the secondary cardiovascular complications associated with uncontrolled hypertension and potential of early target organ damage to increase their motivation for behavior change as well as their knowledge of how to improve BP control. The addition of the PACHT-c pharmacist/APN consultation intervention will integrate improved access and effective approaches to intensify BP medications. TOUCHED evaluates an innovative, integrated approach to improving BP’s in an underrepresented population with uncontrolled/undiagnosed HTN identified in the ED.
Contact
Brenda Lara, MA
blara3@uic.edu -
Building Interdisciplinary Research in Women’s Health/Center for Research on Women and Gender/College of Medicine
Project Description
Building Interdisciplinary Research in Women’s Health/Center for Research on Women and Gender/College of Medicine (BIRCWH/CRWG/COM Program) evaluates sex differences in the pre-hospital, hospital level and survival outcomes, using CARES (cardiac arrest registry to enhance survival), the largest national database of out-of-hospital cardiac arrest. Additionally, the HCHS/SOL database, along with the ancillary study database, Echo-Sol, are being used to establish echocardiographic strain normals in hispanics and explore prediction models using strain for incident heart failure and myocardial infarction. Finally, the study will also explore the potential use of optic nerve sheath diameters (ONSD) using bedside ocular ultrasound in cardiac arrest patients to see if ONSD can be used as a neuroprognostication tool in the post-resuscitation care of cardiac arrest patients.
Who is involved?
- Principal Investigator: Pavitra Kotini-Shah, MD
- Terry Vanden Hoek, MD
- Martha L. Daviglus, MD, PhD
- Pauline Maki, PhD
- Stacie Gellar, PhD
Contact
Pavitra Kotini-Shah, MD
pkotini@uic.edu
Current Research Heading link
- HEED the GAP
- Prevention and Screening of Cancer in the Emergency Department Patient Population (PaCE)
- Project IDEAL
Past Research Heading link
-
COVID Testing Registry (CTR)
The COVID-19 pandemic has taken a toll on Illinois, but if we can better understand the virus, we can fight it more effectively. That is why UI Health is conducting research to understand who develops COVID-19 and why some patients with COVID-19 only have mild illness while others need to be hospitalized. Patients who agree to join the registry will receive a text on their cell phone to answer questions about home and health needs and then again in about 14 days. Patients’ electronic health records will be reviewed to access information related to COVID-19 symptoms and other clinical information. Additionally, patients who access the web link are prompted to choose what types of resources they want to learn about.
Principal Investigator: Marina Del Rios, MD, MSc, Emergency Medicine
Co-Investigators:
- Susan Bleasdale, MD, Infectious Disease, Chief Quality Officer
- Steven Dudek, MD, Pulmonary/Critical Care Medicine
- Pavitra Kotini-Shah, MD, Emergency Medicine
- Jerry Krishnan, MD, PhD, Pulmonary Critical Care Medicine
- Janet Lin, MD, MPH, MBA, Emergency Medicine
- Renee Petzel Gimbar, PharmD, Pharmacy
- Adam Rodos, MD, Emergency Medicine
- Leah Finkel, MD, Emergency Medicine
Project Coordinators:
- Shaveta Khosla, PhD, MPH, Emergency Medicine
- Ruth Pobee, PhD, Emergency Medicine
Aims:
This research registry is being done to better understand the patients who are tested for COVID-19 at UI Health and are discharged home. The Specific Aims for the UI Health CTR are as follows:- Estimate the number of COVID-19 tests per week who are discharged from a UI Health testing location to home, feasibility of obtaining consent for the UI Health CTR, and participant burden.
- Describe the characteristics of COVID-19 tested patients who are discharged from a UI Health testing location to home, including proportion who test positive, socio-demographics, comorbid conditions, current medical treatment (including use of statins, since such patients would not be eligible for a simvastatin-based clinical trial), and health status over a 14-day period.
Study Design
CTR is an observational study. Each participant in the CTR will be followed for 14 days.
Contact
- Shaveta Khosla
skhosl2@uic.edu - Ruth Pobee
rpobee@uic.edu
-
Personalized Analytics and Wearable Biosensor Platform for Early Detection of COVID-19 Decompensation (DeCODe)
DeCODe uses remote monitoring of continuous vital signs at home to try to predict deterioration and re-admission to the hospital. The study is 4 weeks long and involves wearing a medical device patch and using a cell phone we provide to take daily surveys.
Objective:
Collect sufficient data to identify a set of predictor variables that most accurately predict a COVID-19 decompensation event aimed at developing and validating a clinically-useful COVID Decompensation Index (CDI).
Description of Study Intervention:
pinpointIQ™ is a continuous remote patient monitoring system intended for use by healthcare professionals for collection of physiological data. The end-to-end solution consists of the VitalPatch Sensor (a 510k-cleared disposable patch with integrated biosensors and a wireless transceiver) and the physIQ Platform (a mobile application for data transmission, cloud-based information-technology [IT] infrastructure, physiology analytics modules, and clinician user interface). The patch is worn on the torso for up to 7 days and measures and records physiological variables that can include, but are not limited to, electrocardiography (ECG) waveforms, vital signs and activity. Data are transmitted wirelessly from the VitalPatch Sensor to the physIQ IT platform for storage and analysis and presentation within the clinician user interface. A watchlist in the user interface displays alerts that represent clinician defined events. Questionnaires provided through the mobile app can also be responded to by the patient and responses viewed in the clinician portal (user interface).
Study Population:
Participants will be adult patients in the University of Illinois Health System (UIH). Participants will be recruited from two pools of patients at UIH: 1) patients tested in the outpatient setting who have a positive result for SARS-Cov-2 (COVID-19) and 2) patients who were admitted to the hospital with a diagnosis of COVID-19 and subsequently discharged to home convalescence. This will be a convenience sample. Phase 1 will have a sample size of 400 and Phase 2 will have a sample size of 1,200.
Sample Size:
- Phase 1: 400 patients
- Phase 2: 1200 patient
Description of Sites/Facilities Enrolling Participants:
University of Illinois at Chicago (UIC), UIH emergency department and 4 associated outpatient clinics. Also, UIH is comprised of a clinical enterprise that includes a 462-bed tertiary care hospital, outpatient clinics, and Mile Square Health Center locations, which are all Federally Qualified Health Centers (FQHC).
Study Duration: Phase 1: expected 4 months, Phase 2: expected 6 months
Participant Duration: 28 days
Contact
- Andrew Best
azbest2@uic.edu - Janey Kottler
jjubas2@uic.edu
-
Predictors of SEVERE COVID-19 Outcomes (PRESCO)
PRESCO stands for “Predictors of Severe COVID-19 Outcomes”. PRESCO is a longitudinal, multi-center, observational study collecting diverse biological measurements and clinical and epidemiological data for the purpose of enabling a greater understanding of the onset of severe outcomes, primarily acute respiratory distress syndrome (ARDS) and/or mortality, in patients presenting to the hospital with suspicion or diagnosis of COVID-19. We seek to understand whether there are early signatures that predict progression to ARDS, mortality, and/or other comorbid conditions.
The primary objective of the study is to build models that predict the risk of development of ARDS and/or mortality among COVID-19 patients who present to the hospital for evaluation and treatment, to inform prioritization of those individuals at high-risk for earlier and more aggressive intervention. Risk prediction will be based on epidemiological, clinical, cellular, and/or molecular signatures that will be identified in the course of the study. The primary endpoint of the study is performance (discrimination / calibration) of models that predict the risk of development of ARDS and/or mortality among COVID-19 patients who present to the hospital for evaluation and treatment.
Approximately 1500 participants classified as a Person Under Investigation (PUI) for COVID-19 or confirmed positive for COVID-19 will be enrolled from approximately 10 investigational sites. Investigational sites may include satellite locations of a primary site. Participants will be enrolled from the time they present to the hospital until discharge (estimated to be approximately 0-21 days). An optional post-discharge follow-up visit will occur approximately 90 days after discharge.
Site PIs: Dawood Darbar, MD; Patricia Finn, MD; Terry Vanden Hoek, MD; Jeff Jacobson, MD; Jan Kitajewski, PhD; Jerry Krishnan, MD, PhD; Janet Lin, MD, MHA, MBA; Heather Prendergast, MD, MPH, MS;
Contact
- Muriel Chen
yining@uic.edu - Stephanie Tiwari
stiwar2@uic.edu - Lourdes Norwick
lnorwick@uic.edu
- Muriel Chen
-
Community Targeting of Uncontrolled Hypertension
Chicago has some of the highest rates of uncontrolled high blood pressure in the country with these rates being greatest among African American and Hispanic individuals.
The Community Targeting of Uncontrolled Hypertension (CTOUCH) research study is a community based hypertension intervention conducted in predominately minority communities/churches. The primary goal of this study is to identify individuals with high blood pressure (BP >140/90 mmHg) before they develop the secondary complications associated with hypertension such as heart failure and renal failure requiring dialysis. Four community churches with predominantly African American or Latino populations located in the Pilsen, Humboldt Park, Bronzeville and Austin communities were selected because of the high rates of secondary complications from uncontrolled HTN (kidney failure and heart failure) in these communities. Following blood pressure screening, individuals participate in an educational intervention aimed at improving understanding of high blood pressure and empowering them to control their blood pressures.
Contacts
- Heather Prendergast, MD, MS, MPH
hprender@uic.edu - Maya Jackson
mjacks35@uic.edu
- Heather Prendergast, MD, MS, MPH
-
Coordinated Healthcare Interventions for Childhood Asthma Gaps in Outcomes
Chicago is an epicenter for asthma health disparities in the U.S., with African-American children 5-11 years bearing a disproportionate share of the burden. Among the most visible of these disparities is the high rate of visits to the Emergency Department (ED) for uncontrolled asthma. It is unclear how effective guideline recommendations and strategies to reduce environmental triggers of asthma really are after children are discharged from the ED.
The CHICAGO (Coordinated Healthcare Interventions for Childhood Asthma Gaps in Outcomes) Plan is a collaborative effort between 13 Chicago based institutions, including the University of Illinois Hospital & Sciences System. This broad-based collaborative, including caregivers, patient advocacy groups, public health officers, and patient-centered outcomes researchers, is dedicated to eliminating asthma health disparities. The CHICAGO Plan tests both provider- and patient-level interventions to improve clinically meaningful outcomes in a minority pediatric ED population with uncontrolled asthma. The goal is to improve outcomes in minority children with uncontrolled asthma through provider and patient-level interventions.
Contact
- Trevonne Thompson, MD
tthomps@uic.edu
- Trevonne Thompson, MD
-
Illinois Heart Rescue US- US Heart Rescue Consortium (Phase II)
Illinois Heart Rescue (ILHR) seeks to increase out-of-hospital cardiac arrest (OHCA) survival rates in the State of Illinois by at least 100%. To accomplish this goal, we have partnered with the Illinois Department of Public Health and existing state-wide quality improvement systems (Get with the Guidelines Stroke, Mission Lifeline) to recruit and train Emergency Medical Services (EMS) systems and hospitals in Illinois to collect quality cardiac arrest data into the CARES database that encourages improvement in local systems of care for sudden cardiac arrest. Our focus is to reduce health disparities in cardiac arrest outcomes using hot spotting to identify and intervene in communities with the highest incidence of cardiac arrest and poorest outcomes.
Since 2013, ILHR has trained over 24,300 community members on Hands Only CPR and AED use through establishing strong partnerships via diverse trainings at schools, hospitals, health associations, cultural centers, and major league sporting events, primarily in identified “hotspot”neighborhoods that have been documented to have the highest incidence of OHCA and the lowest incidence of bystander CPR. ILHR training efforts have resulted in bystander CPR rates in Chicago increasing from 13% in 2013 to 24% in 2015. In this time, Illinois has doubled its survival rate from 4% to 8%.
Contacts
- Courtney Schwerin, Executive Director
courtney.ILHR@gmail.com
- Courtney Schwerin, Executive Director
Past Research Heading link
- Racial Ethnic and gender disparities Among a COVID-19 hyperTensive population (REACT)
- RECOVER
- A Hypertension Emergency Department Intervention Aimed at Decreasing Disparities (AHEAD2)
- Emergency Patient Interdisciplinary Care Coordination for Frequent ER Visitors (EPIC)
- Heart Rescue India
- Improving Sickle Cell Care in Adolescents & Adults in Chicago (ISAAC)
- ICARE (Improving Cancer Survival and Reducing Treatment Variations with Protocols for Emergency Care)
- IPHI- Alliance for Health Equity Hospital Opioid Treatment and Response (HOTR) Demonstration Project
- Lightning Injury Research Program
- Lain@s Gaining Access to Networks for Advancement in Science (L@S GANAS mentorship program)
- Measuring Disparities in the Chain of Survival among Hispanic Communities
- NIH- ROOTS- Reaching Out Of The System
- PMV Sleep Study (The Impact of Sleep Deprivation on Reaction Time Measured by Psychomotor Vigilance Testing in The Emergency Department)