Veiga-Lopez Lab Research
Placental Toxicology Heading link
The placenta plays a critical role in fetal development by regulating nutrient transfer, hormone production, and acting as a barrier to environmental exposures. Our research explores how endocrine-disrupting chemicals (EDCs) affect placental function (Gingrich et al., Trends in Endocrinology & Metabolism 2020). We have discovered that bisphenol S (BPS), the second most prevalent bisphenol, interferes with epidermal growth factor receptor (EGFR) signaling (Ticiani et al., Chemosphere 2023). EGFR signaling is essential for regulating trophoblast cell functions such as proliferation, invasion, and fusion, which are crucial for placental development and the establishment of the maternal-fetal interface (Waye et al., Toxicological Sciences et al, 2025).
Our work has shown that bisphenol S, an emerging bisphenol, reduces the endocrine capacity of the placenta (Gingrich et al., Archives of Toxicology, 2018) and that it can cross the placental barrier reaching fetal circulation (Gingrich et al., Chemosphere, 2019). Our pregnancy PBPK model shows that BPS is more likely to accumulate in the fetal compartment compared to BPA (Gingrich et al., Environment International 2021). In vitro, BPS impairs the fusion and invasion of human cytotrophoblasts (Ticiani et al., Environmental Health Perspectives, 2021 & Ticiani et al., IJMS, 2022). We have demonstrated this in a novel 3D microfluidic platform designed to mimic the placental environment and provide more accurate insights into placental cell behavior (Pu et al., Lab on a Chip, 2021). Our most recent work also shows that EGFR-interfering chemical mixtures disrupt mitochondrial function and bioenergetics in placental cells, revealing potential pathways by which chemical exposure may compromise placental health (Waye et al., Chemosphere, 2024 and Waye _blanket al., Toxicology and Applied Pharmacology 2024).
We are continuing this work through an interdisciplinary collaboration with the Microfabricated Tissue Models lab to develop a state-of-the-art 3D hepatic-placental tandem organ on a chip for high-throughput toxicological screening. This platform will simulate liver metabolism, enabling us to assess how chemicals affect placental function after being processed in the liver, advancing our ability to evaluate reproductive toxicity.
We are expanding our research through a new Virtual Consortium for Translational/Transdisciplinary Environmental Research (ViCTER) interdisciplinary collaboration with Michigan State University and Emory University focusing on how environmental chemicals impact the placental molecular clock. Our ongoing work investigates the role of the epidermal growth factor (EGF) signaling pathway in regulating the placental clock and how chemical mixtures may disrupt this process, leading to placental dysfunction and contributing to adverse reproductive outcomes.
This research is supported by a NIH R01, an NIH ViCTER R01, and an R21 award from the NIEHS.
Bisphenol S reduces e-cadherin expression in the placenta (Arch Tox 2018) Heading link
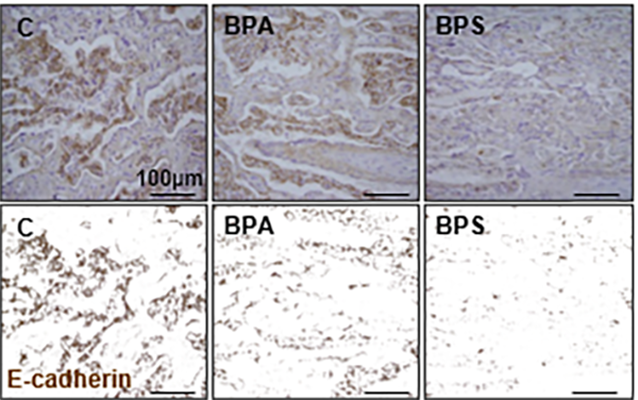
Bisphenol S reduces e-cadherin expression in the placenta (Arch Tox 2018)
3-D microfluidic system for placenta trophoblast cell invasion and cell-to-cell interaction studies under dynamic environment conditions (Lab on a Chip, 2021) Heading link
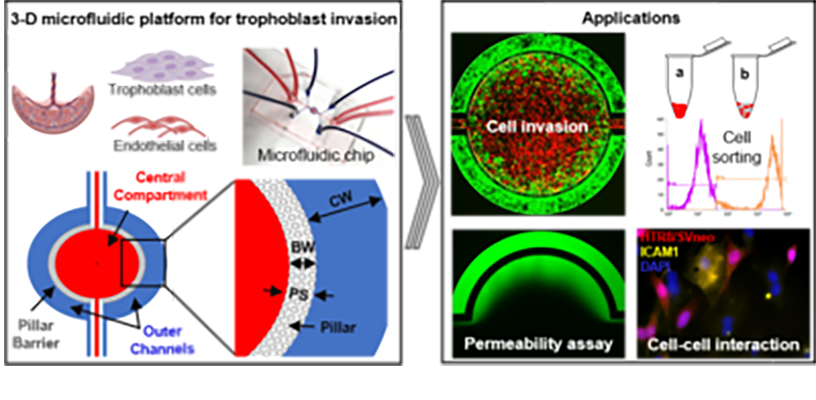
3-D microfluidic system for placenta trophoblast cell invasion and cell-to-cell interaction studies under dynamic environment conditions (Lab on a Chip, 2021)
BPS blocks EGF-mediated human cytotrophoblast (hCTB) cell fusion (Env. Health Perspect. 2021) Heading link
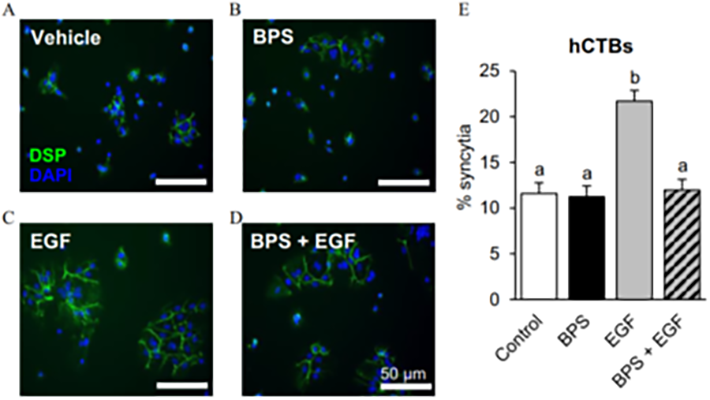
BPS blocks EGF-mediated human cytotrophoblast (hCTB) cell fusion (Env. Health Perspect. 2021)
Super-resolution dSTORM images of mitochondria of human extravillous trophoblasts cells after exposure to EGF or the chemical mixture (Waye et al., Chemosphere 2024). Heading link
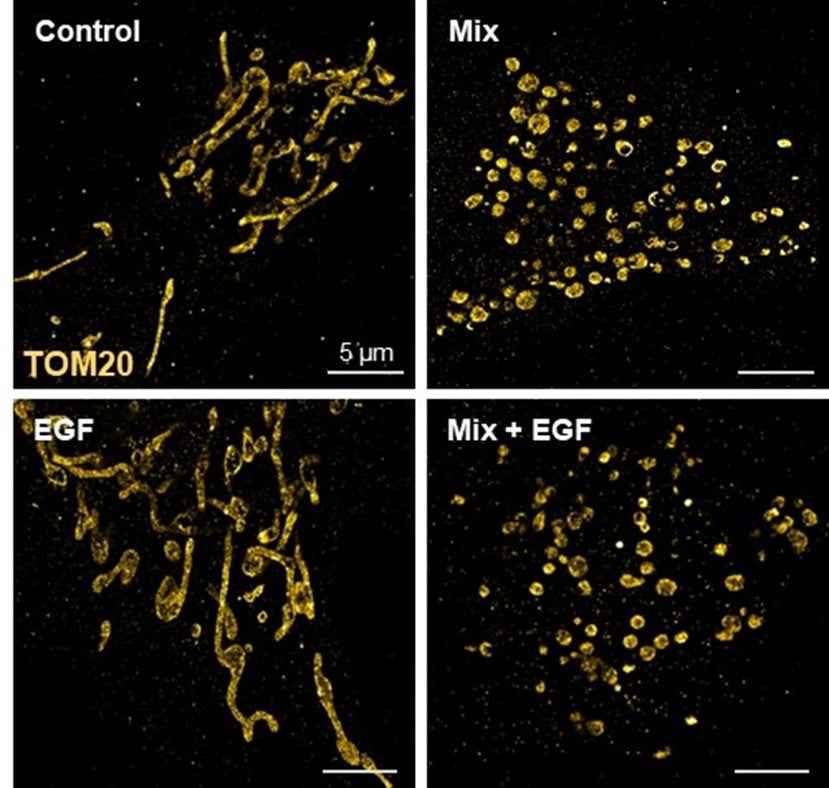
Super-resolution dSTORM images of mitochondria of human extravillous trophoblasts cells after exposure to EGF or the chemical mixture (Waye et al., Chemosphere 2024).
Ovarian Toxicology Heading link
Our research explores how chemicals that interfere with lipid metabolism, known as obesogens, impact ovarian health. We have demonstrated that obesogens, including organotins such as tributyltin (TBT) and triphenyltin (TPT) disrupt cholesterol trafficking and induce lipid dysregulation in ovarian theca cells. These chemicals by altering lipid-related gene expression and increasing lipid content in ovarian cells (Pu et al., Archives of Toxicology 2019; Pu et al., Toxicology and Applied Pharmacology, 2022; Pascuali et al., Environmental Health Perspectives, 2024). Using MALDI-TOF, we have also recently mapped the impact of TBT on the ovarian lipidome, revealing region-specific disruptions in lipid species that may contribute to infertility and reproductive dysfunction (Pascuali et al., Environment International, 2024).
Neutral lipid droplets in primary human theca cells exposed to tributyltin (Environmental Health Perspectives, 2024) Heading link
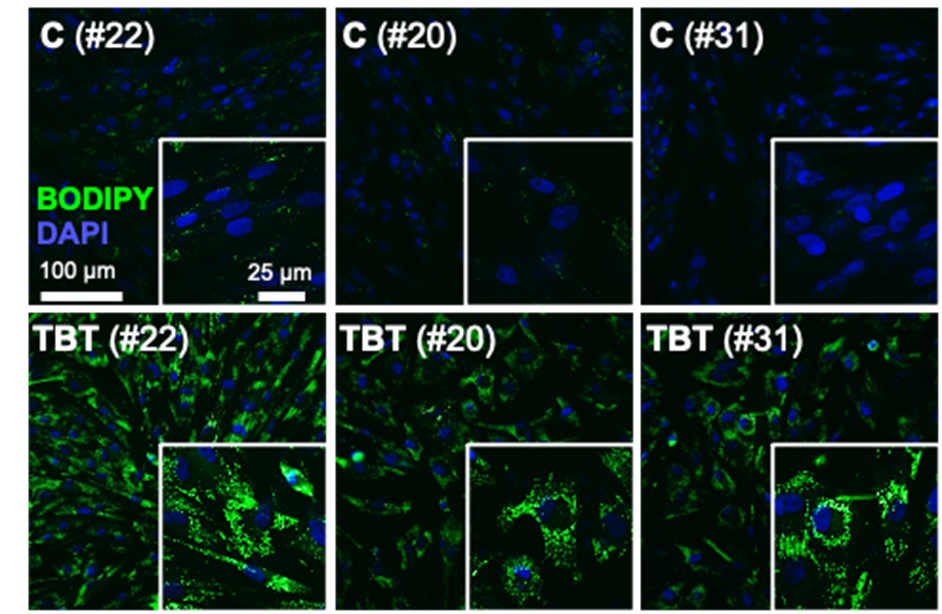
Neutral lipid droplets in primary human theca cells exposed to tributyltin (Environmental Health Perspectives, 2024)
Ion images generated by MALDI MSI of mouse ovarian sections reveal spatial differences matching functional regions (Env Int, 2024) Heading link
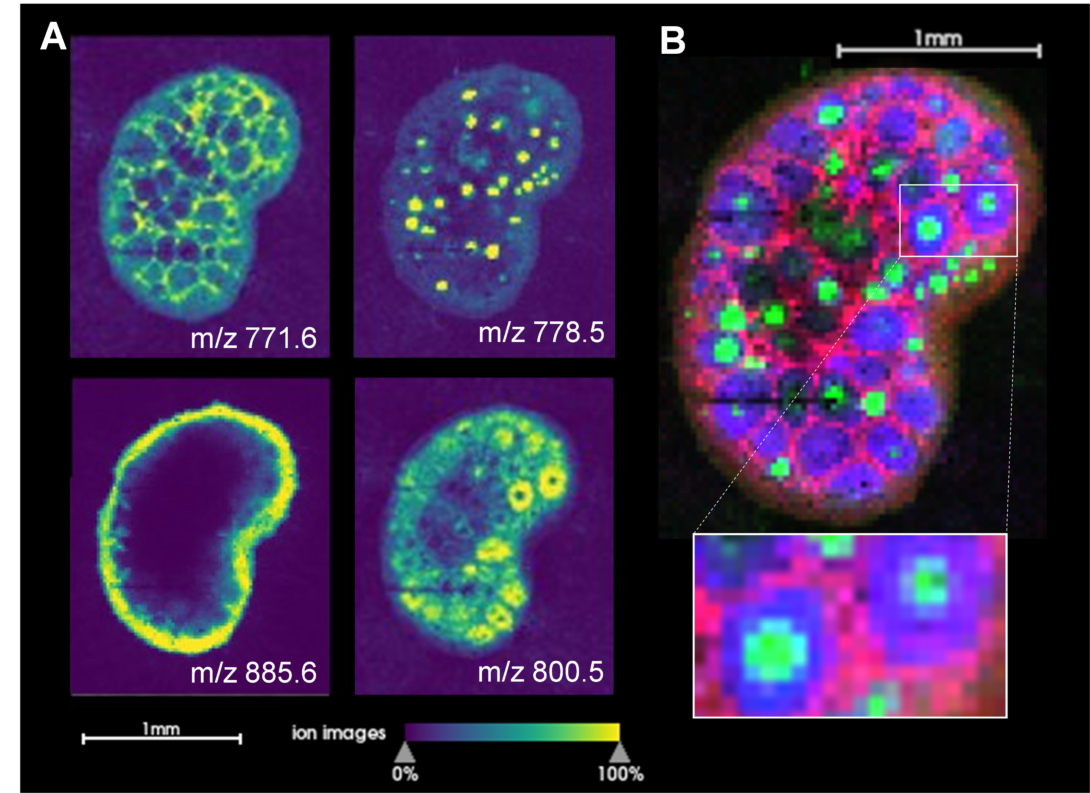
Ion images generated by MALDI MSI of mouse ovarian sections reveal spatial differences matching functional regions (Env Int, 2024)
Fetal origins of metabolic disease Heading link
Fetal exposure to the endocrine disrupting chemical bisphenol A (BPA) causes insulin resistance in the adult (Veiga-Lopez et al., Am J Physiol – Endocrinol Metab 2016), a pre-stage of type 2 diabetes, which affects ~350 million people. In women, gestational BPA exposure affects the newborn’s weight in a sex-specific manner (Veiga-Lopez et al., J Clin Endocrinol Metab 2015). Animal studies suggest that weight increases are due to increase body fat. How BPA induces insulin resistance and weight gain/obesity remains unknown. Our research using in vivo and cell-based assays aims to understand the mechanistic basis by which BPA programs insulin resistance and obesity during fetal life. Our work has demonstrated for the first time that gestational BPA can increase the ability of fetal primary preadipocytes to differentiate into mature adipocytes and points to the endoplasmic reticulum stress as a potential modulator in this effect. (Pu et al., Endocrinology 2017). Our recent work has also focused on another insulin target tissue, the skeletal muscle. We have demonstrated sex- and bisphenol-specific effects on myocytes upon prenatal exposures (Jing et al., Toxicological Sciences 2020).
Check out our review on the effects of endocrine disrupting chemicals on adipogenesis (Obesogenic endocrine disrupting chemicals: identifying knowledge gaps. Trends in Endocrinology & Metabolism 2018).
This work was funded by an NIEHS/NIH K22 TIEHR award.
Gestational BPA leads to increased fetal adipogenic differentiation. Heading link
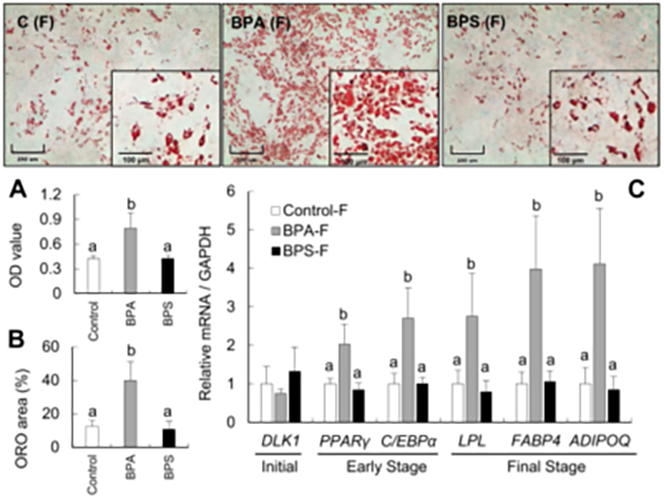
Gestational BPA leads to increased fetal adipogenic differentiation.
BPA and BPS alters myogenic differentiation during fetal life. Heading link
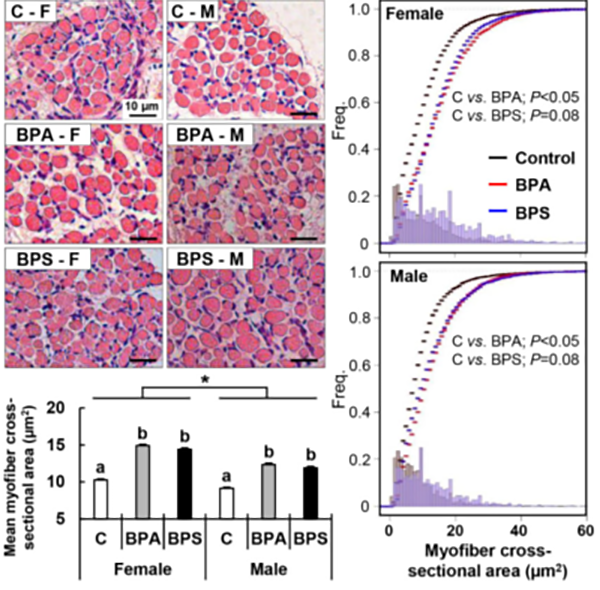
BPA and BPS alters myogenic differentiation during fetal life.
Improving small ruminants intensive production systems Heading link
As a veterinarian, I also have a substantial interest in applied research to improve sheep production systems, an outstanding priority for the Michigan Sheep Breeders Association. Given how maternal nutritional status and environment affect offspring health, my laboratory is partnering with Dr. Richard Ehrhardt (Michigan State University) and conducting studies focusing on the effects of plane of nutrition and its relationship with breeding season in an intensive sheep production system at the Sheep Teaching & Research Center. Some of our work relates to placental endocrine function (Roberts et al., Theriogenology 2017), effects of production practices on placental function (Rosales-Nieto et al., 2020) and how maternal nutrition affects placental function and offspring growth (Rosales-Nieto et al., 2021).
This work is currently funded by the Michigan Alliance for Animal Agriculture.
Dynamic change in pregnancy associated glycoproteins (PAG1 & PSBP) through pregnancy (Roberts et al., 2017) Heading link
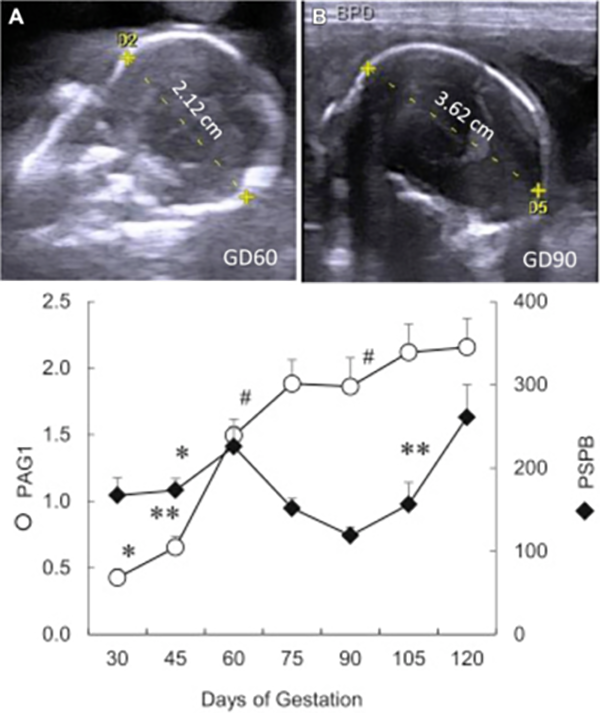
Dynamic change in pregnancy associated glycoproteins (PAG1 & PSBP) through pregnancy (Roberts et al., 2017)
Pregnancy associated glycoproteins reach baseline 10 weeks postpartum in the mother and less than10 days in the newborn (Roberts et al., 2017) Heading link
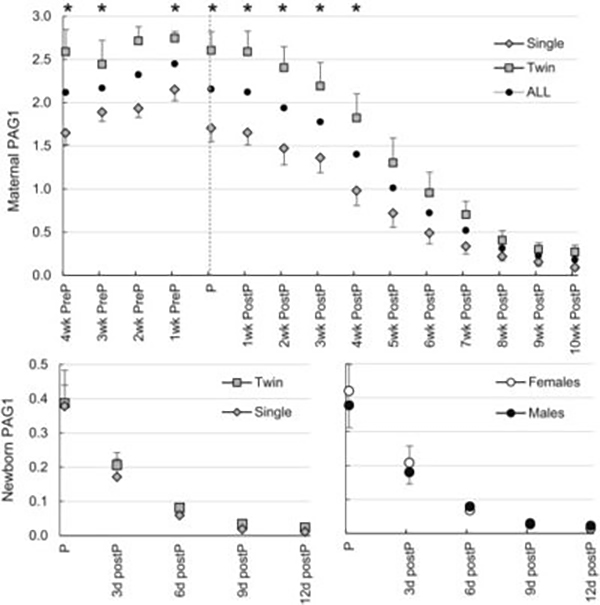
Pregnancy associated glycoproteins reach baseline 10 weeks postpartum in the mother and less than10 days in the newborn (Roberts et al., 2017)