
Sojin Shikano, PhD
Associate Professor
Department of Biochemistry and Molecular Genetics
Contact
Office Phone:
Lab
Building & Room:
312-996-2091
Email:
Related Sites:
Research Interests Heading link
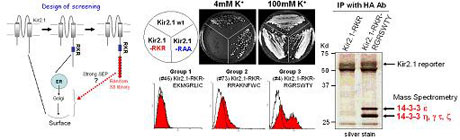
Molecular interactions directing the membrane protein localization My laboratory is interested in understanding the molecular mechanisms of the membrane protein trafficking. As the human genome sequences have become available, the mutations of genes that cause many human diseases are correlated with abnormal intracellular trafficking. The mechanisms for controlling the protein targeting are therefore of great interest both conceptually and clinically. Currently we focus on identifying and mechanistically characterizing the transport signals on cargo proteins that ‘zipcode’ the surface membrane localization. Our long term goal is to provide a basis for future therapeutic intervention of disorders arising from the protein trafficking defect.Protein sorting is often mediated by short peptidic epitopes,such as the C-terminal KKXX for endoplasmic reticulum (ER) residential transmembrane proteins and the internal ‘RXR’ signals found in multimeric transmembrane proteins. These signals serve as the recognition signatures for transport machinery proteins that are responsible for retrieval of cargo vesicles back to ER. For cell surface expression of membrane proteins, the export from ER is the first critical step. Earlier it was believed that the cell surface proteins can automatically exit ER by ‘bulk-flow’ mechanism unless they are trapped by ER retention/retrieval machineries as a result of misfolding or misassembly. However, recent works have revealed the presence of specific ‘forward trafficking’ signals that actively mediate ER export in some proteins.
One of the important emerging themes is that a given surface membrane protein may contain multiple different sorting signals, such as ER retention/retrieval and ER export. Such signal organization in a protein permits a molecular mechanism of multiple check points for posttranslational events that include folding, assembly, and modifications. Obviously, the interesting question here will be: “what is the mechanism that organizes multiple different motifs in an individual molecule and eventually confers the proper localization at cell surface?” In order to understand this, the comprehensive approach to the identity of ER export signals is essential. So far, however, most of such signals have been found as results of studies on individual proteins.
We have embarked on a systematic search for the forward trafficking signals in membrane proteins. We developed a gain-of-function screening using the power of yeast genetics, and identified a number of novel peptide signals that direct surface expression of membrane proteins in mammalian cell. These include a group of sequences that conferred surface expression by overriding the ER retention signal through specific interaction with 14-3-3 proteins in a phosphorylation-dependent manner. This would represent a new protein targeting pathway by which the cells respond to the external signals involving phosphorylation. We are currently studying the molecular mechanisms underlying this 14-3-3-mediated surface expression of membrane proteins by genetic and biochemical approach.
Our research interests include:
- Screening and functional characterization of novel protein transport signals
- Molecular mechanisms for 14-3-3-mediated surface expression
- Regulatory mechanisms of ‘RXR’ ER retention/retrieval signal
- Signal-induced transport of potassium channels
- Utilization of host transport machineries by infectious pathogens
Education
PhD, DVM, University of Tokyo
Post Doctoral Fellow UT Southwestern Medical Center John Hopkins University