
Pradip Raychaudhuri, PhD
Professor
Department of Biochemistry and Molecular Genetics
Contact
Office Phone:
Lab
Office Phone:
Email:
Related Sites:
Major Interests Heading link
Mechanisms of tumor metastasis by FoxM1:
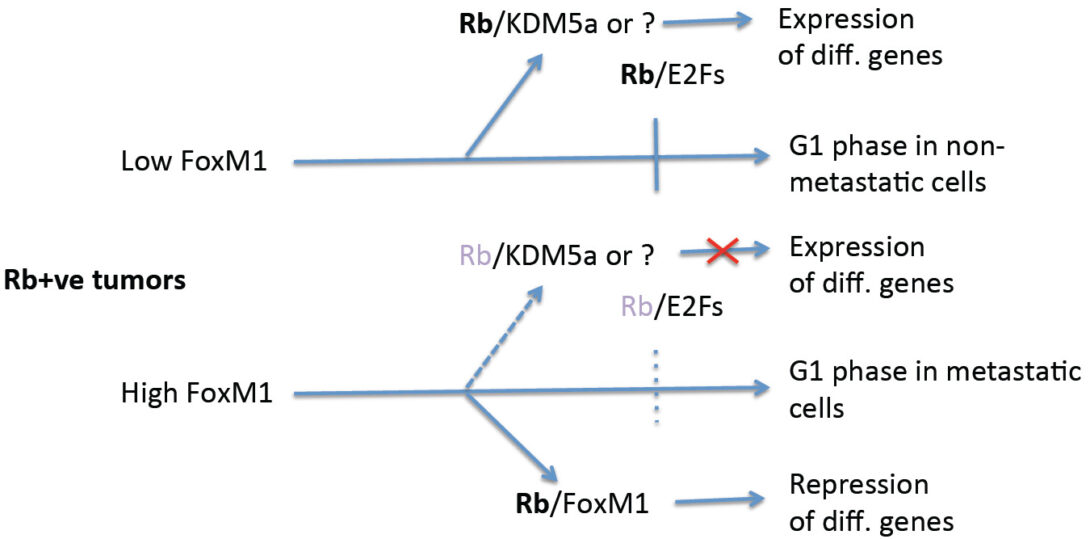
Metastasis is often the main cause of fatality from cancer. Despite significant progresses on the steps involved in metastasis of tumor cells from a primary site to distal organs, our understanding of the molecular details of how tumor cells evolve and acquire the ability to metastasize is sketchy. Some progress has been made on the metastatic cells. For example, cluster of disseminating tumor cells expressing basal epithelial markers have been implicated in metastasis of breast cancer. Other studies indicated involvement of the cancer stem-like cells in metastasis. We focus on the fork-head box transcription factor FoxM1 that is over-expressed in aggressive types of breast cancer. Current studies are based on our new findings, which surprisingly indicate that FoxM1’s interaction with the retinoblastoma (Rb) family proteins is important for tumor metastasis. The central hypothesis is that, the gene-repression function of FoxM1, involving the retinoblastoma family proteins, plays critical roles in the progression of metastatic breast cancer.
FoxM1 stimulates proliferation/pro-cancer genes and represses differentiation genes. We were able to genetically dissect the repressor function from the activation function. Phosphorylation of FoxM1 by Plk1 converts a transcriptional repressor form of FoxM1 to an activator form. In the absence of Plk1-phosphorylation, FoxM1 binds to Rb to function as a repressor. Plk1 phosphorylated-FoxM1 does not bind Rb, instead it binds to the co-activator CBP. Consistent with that, a Plk1-sites phospho-mimetic mutant of FoxM1 (FoxM1 DD) activates transcription, but fails to bind Rb or to repress the differentiation gene GATA3.
Using the CRISPR/Cas9 technology, we have generated a knock-in mouse strain that expresses the repression-deficient mutant of FoxM1 (FoxM1DD). Unlike the FoxM1-/- mice, there is no overt developmental defect, except that the females exhibit deficiencies in lactation. We are investigating whether the lactation deficiency is related to an early loss of mammary luminal progenitor cells in the FoxM1 DD/DD females. Interestingly, the FoxM1DD/DD mice support MMTV-PyMT-driven development of mammary tumors as efficiently as FoxM1 +/+ mice, but the tumors in FoxM1 DD/DD mice are severely deficient in metastasis. Single-cell RNA-Seq analyses revealed that the tumors in the FoxM1 DD/DD background are mainly of differentiated type and they contain fewer cancer stem-like cell (as predicted from the model below). We are using this mouse model and a model for basal-like tumors to further investigate how gene repression function of FoxM1 drives metastasis.
Honors & Awards Heading link
UIC University Scholar (2011-2014)
1992 Pew Biomedical Scholar
Education
PhD, Albert Einstein College of Medicine
Postdoctoral, Duke University