
Toru M. Nakamura, PhD
Professor
Department of Biochemistry and Molecular Genetics
Pronouns: He/Him
Contact
Building & Room:
MBRB Room 2202
Address:
900 S. Ashland Ave. MC669 Chicago IL 60607
Office Phone:
Lab
Building & Room:
MBRB Room 2206
Office Phone:
Email:
Related Sites:
Research Interests Heading link
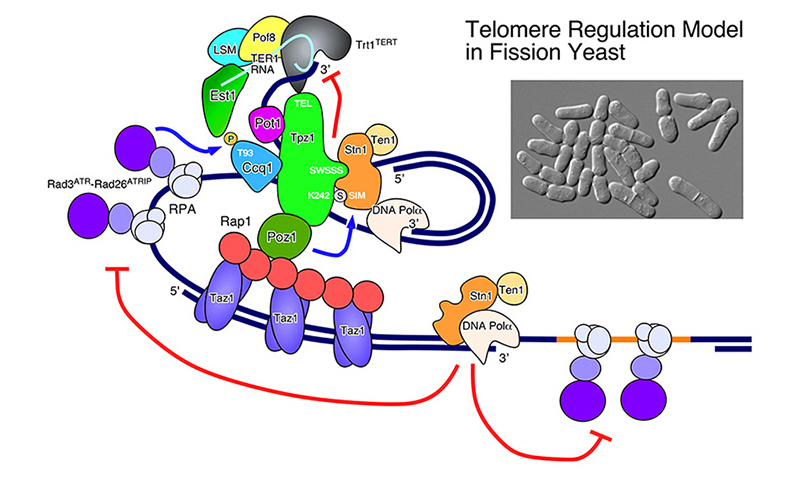
Our laboratory is interested in understanding how checkpoint and DNA repair proteins contribute to maintenance of telomeres, the natural ends of linear eukaryotic chromosomes. Proper maintenance of telomeres is crucial for stable inheritance of the genome. Various checkpoint and DNA repair proteins, including evolutionarily highly conserved checkpoint kinases Tel1 (ATM) and Rad3 (ATR), play important roles in stable maintenance of telomeres. However, mechanistic roles for various checkpoint and DNA repair proteins in telomere maintenance have not been fully established. Deregulation of telomere maintenance mechanisms has been found to be a key event in tumorigenesis, thus mechanistic insights on how various proteins collaborate to generate functional telomeres might lead to effective methods for preventing cancer.
We use fission yeast Schizosaccharomyces pombe as a model system. Advantages of S. pombe include well-characterized DNA damage responses with high structural and functional conservation to mammalian cells, and amenability to genetic, biochemical and cytological studies. In addition, the ability of fission yeast to bypass the need for functional telomere maintenance mechanisms by circularizing all chromosomes provides flexibility, not available in any other organisms, in manipulating telomere related genes without being hindered by cell lethality. In fact, S. pombe cells lacking telomerase, as well as cells lacking both Tel1ATM and Rad3ATR kinases survive telomere maintenance defects by circularizing all three chromosomes.
We have demonstrated by quantitative chromatin immunoprecipitation assays that the leading strand DNA polymerase (Pol ε) arrives to replicating telomeres significantly earlier than the lagging strand DNA polymerases (Pol α and Pol δ), and replicating telomeres strongly recruit Replication Protein A (RPA) and Rad3-Rad26 (ATR-ATRIP) complexes in fission yeast. We have also established the cell-cycle-regulated recruitment timing for MCM, Mre11-Rad50-Nbs1 (MRN) complex, Trt1 (TERT, catalytic subunit of telomerase), and telomere capping proteins (Pot1 and Stn1). In addition, we have established that Tel1ATM and Rad3ATR kinases are redundantly required to promote telomere protection and telomerase recruitment by promoting efficient recruitment of the telomere capping complex subunit Ccq1 to telomeres.
We have also discovered that Tel1ATM/Rad3ATR-dependent phosphorylation of Ccq1 on Thr93 is essential for telomerase association with telomeres, and that the 14-3-3-like domain of the telomerase regulatory subunit Est1 specifically recognizes and binds the phosphorylated Thr93 of Ccq1. Phosphorylation of Ccq1 is negatively regulated by the telomerase inhibitors Taz1, Rap1 and Poz1, and telomere elongation and increased telomerase association with telomeres found in rap1Δ cells are dependent on Ccq1 Thr93 phosphorylation. On the other hand, Ccq1 Thr93 phosphorylation is also increased as telomeres shorten in telomerase mutant cells. Taken together, we thus uncovered the Tel1ATM/Rad3ATR-dependent Ccq1-Est1 interaction as a critical regulatory mechanism that ensures stable maintenance of telomeres in fission yeast cells.
Selected Grants
NIH (1R01GM143316), Regulation of Telomere Maintenance in Fission Yeast (https://reporter.nih.gov/project-details/10519426), PI
Selected Publications
Mennie AK, Moser BA, Hoyle A, Low RS, Tanaka K, Nakamura TM. Commun Biol. 2019 2:297.
LARP7-like protein Pof8 regulates telomerase assembly and poly(A)+TERRA expression in fission yeast.
Mennie AK, Moser BA, Nakamura TM. Nat Commun. 2018 9(1):586.
Moser BA, Raguimova ON, Nakamura TM. Mol Biol Cell. 2015 26(21):3857-66.
Harland JL, Chang YT, Moser BA, Nakamura TM. PLoS Genet. 2014 10(10):e1004708.
Miyagawa K, Low RS, Santosa V, Tsuji H, Moser BA, Fujisawa S, Harland JL, Raguimova ON, Go A, Ueno M, Matsuyama A, Yoshida M, Nakamura TM, Tanaka K. Proc Natl Acad Sci U S A. 2014 111(16):5950-5.
Fission yeast shelterin regulates DNA polymerases and Rad3ATR kinase to limit telomere extension.
Chang YT, Moser BA, Nakamura TM. PLoS Genet. 2013 9(11):e1003936.
Tel1ATM and Rad3ATR kinases promote Ccq1-Est1 interaction to maintain telomeres in fission yeast.
Moser BA, Chang YT, Kosti J, Nakamura TM. Nat Struct Mol Biol. 2011 18(12):1408-13.
Telomeres avoid end detection by severing the checkpoint signal transduction pathway.
Carneiro T, Khair L, Reis CC, Borges V, Moser BA, Nakamura TM, Ferreira MG. Nature. 2010 467(7312):228-32.
Publication Aggregators
Notable Honors
2005-2007, Scholar Award, Sidney Kimmel Foundation for Cancer Research
Education
Post-Doctoral Training, The Scripps Research Institute, La Jolla, CA (Advisor: Dr. Paul Russell)
PhD, University of Colorado at Boulder (Advisor: Dr. Thomas R. Cech)
BS, University of Illinois at Urbana-Champaign